HLP003
HLP003:
Deuterated Novel Serotonergic Agonist Program with FDA Breakthrough
Therapy Designation
HLP003 is a proprietary, deuterated novel serotonergic agonist designed to target the 5‑HT2A receptor. It is structurally related to endogenous serotonin and is being developed to harness its targeted receptor activity and pharmacodynamic properties for therapeutic benefit.
HLP003 is a new chemical entity (NCE) and has been granted FDA Breakthrough Therapy Designation for the adjunctive treatment of Major Depressive Disorder (MDD).
Developmental Status: Dosing underway in Helus Pharma’s Phase 3 PARADIGM program in participants with moderate to severe MDD.
Upcoming Milestones: APPROACH topline data in Q4 2026.
PARADIGM:
HLP003 Phase 3 Pivotal Program in MDD

The pivotal program will consist of 2 studies plus an extension study:
• APPROACH: Two-arm study of two doses of HLP003 vs. placebo
• EMBRACE: Three-arm study with a high dose, mid-dose, and placebo arm
• EXTEND: Long-term extension study that allows for open-label dosing or re-dosing for participants who did not respond in the first two studies or relapsed during the extension study
Expanded Access Policy
We do not offer expanded access at this time.
Discover Helus’ complete Research and Development Progress.
To pioneer a paradigm shift in mental health, where novel serotonergic agonists empower durable improvements in healing.
HLP003 received the first known Breakthrough Therapy Designation for an adjunctive NSA therapy for MDD
This designation acknowledges the significant unmet medical need for more effective treatment of MDD. It also provides validation that Phase 2 data for HLP003 shows preliminary evidence for significant clinical improvements over existing therapies.
Breakthrough Therapy Designation expedites the development pathway for HLP003 with the following benefits:
- All Fast Track Designation features, including Rolling Review for NDA
- More intensive FDA guidance and discussion, including planned clinical trials and plans for expediting manufacturing development strategy
- HLP003 is eligible for Priority Review and Accelerated Approval
HLP003 is in development as an adjunctive treatment, with the potential to offer the following benefits:
- Allows for immediate treatment without waiting to withdraw from background medications
- Limits withdrawal symptoms since patients do not need to stop current antidepressant therapy
- Eliminates logistical hurdles associated with titrating off existing medications
- Background medications could provide some benefit even if inadequate alone
Clinical Validation for HLP003 in MDD:
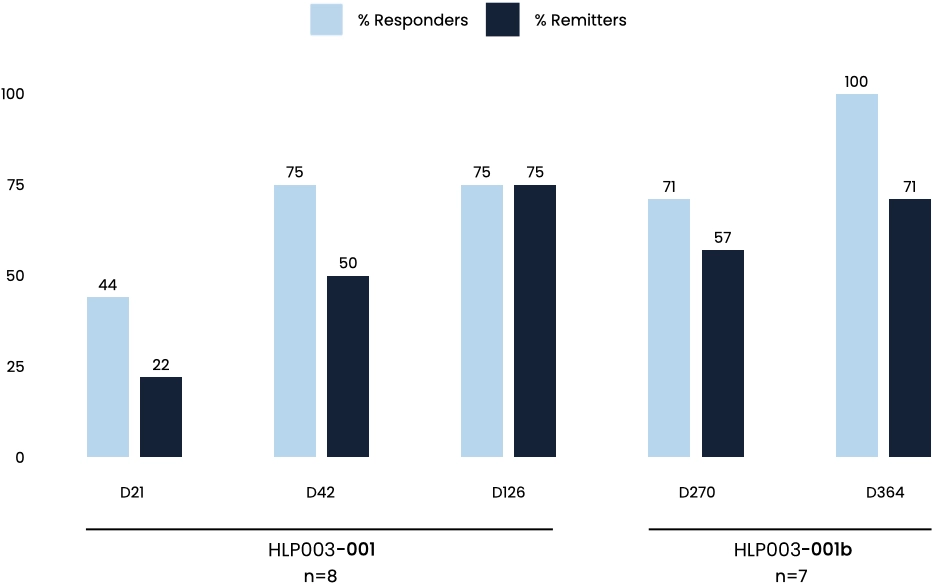
Robust and Durable Remission Rates up to 12 Months
12 month data highlights, after 2 doses of HLP003 (16 mg):
Average baseline MADRS score was ~32
100% of patients were responders
71% of patients were in remission
All reported AEs were mild to moderate.
No AEs/SAEs reported in the 12-month follow-up
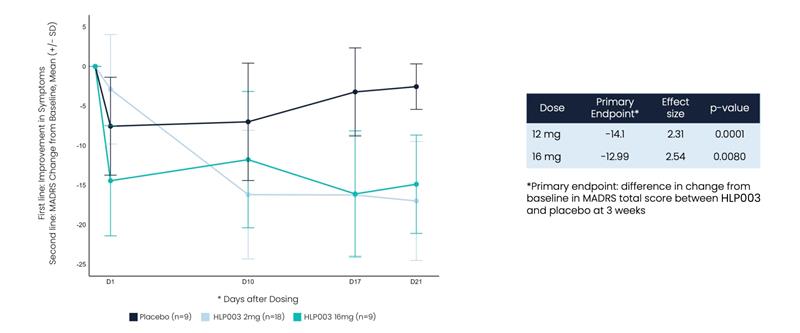
Large Improvement in Depression Symptoms After a Single Dose of HLP003
Response and Remission at 12 Months: 12 mg & 16 mg